HemaTrax®-CT
ISBT 128 compliant labeling software for Cellular TherapyHemaTrax®-CT v3.7.2
Standalone System
Now is the time to implement a simple, on demand print solution for ISBT 128 labeling. Use our HemaTrax®-CT cellular therapy product labeling system to ensure your facility meets these standards.
The new edition (version 8.1) of the FACT-JACIE International Standards for Hematopoietic Cellular Therapy was published December 14, 2021. Please note implementation of ISBT 128 labeling is required for FACT-JACIE accreditation effective May 20, 2018. Don’t be ISBT 128 non-compliant! Implementation of our HemaTrax®-CT system includes a printer, proprietary software, ribbons and labels— all required for ISBT 128 compliance. There are two ways to use HemaTrax-CT. You can run it standalone, meaning no other software is required and it can integrate with cellular therapy systems shown below. You will receive access to our easy to use software. By entering your label information on just a few screens, you can simply and quickly print cellular therapy identification labels.

New ISBT 128 Standard from ICCBBA, January 2023
click to learn more:
ST-028 Chain of Identity Identifier (CoI)
The new ISBT 128 Standard for the Chain of Identity (CoI) Identifier (ST-028), developed based on requirements developed collaboratively with the Standards Coordinating Body (www.standardscoordinatingbody.org) (SCB), has been published. John Kling, President and Jeff Dragoo, CTO of Digi-Trax, both serve on the ICCBBA’s Cellular Therapy Coding and Labeling Advisory Group.
There are circumstances in the collection and processing of cellular therapy products for further manufacture where more than one donation may need to be collected to deliver a given therapy. This new identifier provides a standardized, globally unique identifier that can be used to link these donations together. The CoI Identifier should be allocated either before, or at the time of, collection of the first donation.
The CoI Identifier data structure was also added to ISBT 128 Standard Technical Specification (ST-001), and the label examples in ISBT 128 Standard Labeling of Collection Products for Cellular Therapy Manufacturers (ST-018) have been updated. All ISBT 128 standards and implementation guides are available to the public in the technical library on the ICCBBA website (www.isbt128.org).
ICCBBA thanks all who contributed to this effort, either by participating with the SCB, serving on ICCBBA’s Cellular Therapy Coding and Labeling Advisory Group or Standards Committee, or providing comments during the public comment period.
Our HemaTrax®-CT Cellular Therapy labeling software supports the ICCBBA ST-028 CoI standard. When your facility is ready to implement CoI, HemaTrax®-CT is ready to support your CT labeling efforts.
For more information on this standard, visit ICCBBA’s ST-028 web page.
FACT Encourages Adoption of New ISBT 128 Split Label for Collection for Further Manufacturing
Learn why FACT is enouraging accredited facilities to participate in clinical trials and provide advanced celllar therapies to their patients. Handwritten information create risks of product mix-ups with incorrect information. Learn more
At right, read how our MFG label format can be used for your CT clinical trial “split labeling” solution.
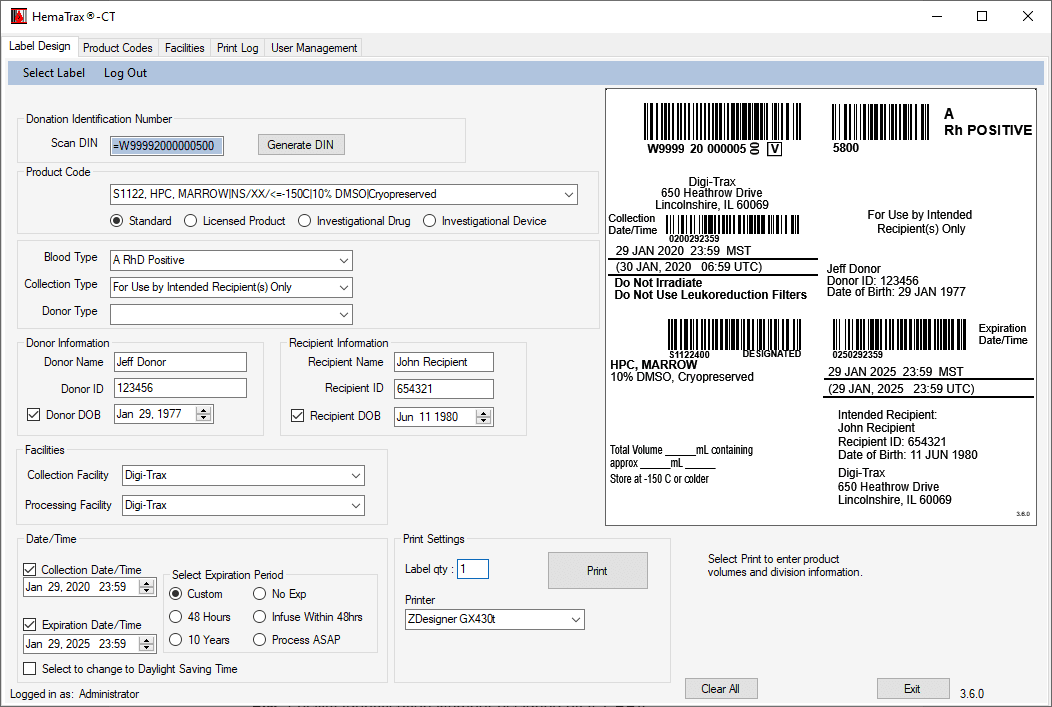
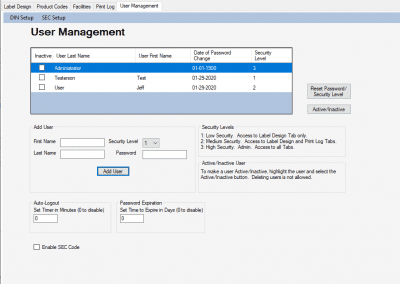
HemaTrax®-CT Standalone software screenshots:
Creating a label with HemaTrax®-CT v3.7.2
HemaTrax®-CT v3.7.3 is now available!
Highlights of v3.7.3 include new ICCBBA product codes v7.72.1, improvements to the MFG label format, along with several upgrades and feature enhancements.
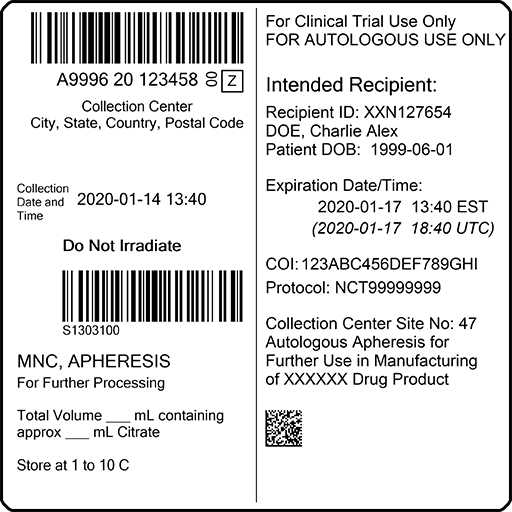
HemaTrax®-CT MFG label format
click to learn more:
HemaTrax®-CT-MFG format included with v3.7.1
HemaTrax-CT 3.7.1 features a new ISBT 128 label format, often referred to as a “split label” format created for cellular therapy clinical trials and manufacturing. This format includes all information required by existing ISBT 128 standards on the left side, and allows manufacturer-specific information on the right side. HemaTrax-CT software includes the ISBT 128 compliant MFG label format that accommodates the “split label” apheresis collection product label format.
The MFG label format is designed specifically for clinical trial sponsors, manufacturers, staff at clinical trial facilities, apheresis collection centers and hospitals that conduct clinical trials or receive manufactured products.
If you purchase a new HemaTrax®-CT system, this format is included. If you have an existing HemaTrax®-CT system, you can purchase the CT-UPGRADE-MFG module separately. Just contact our customer service team at 800-356-6126 or at info@digi-trax.com.
HemaTrax-CT Brochure
View info and label format samples
HemaTrax-CT Folleto (Spanish)
Ver información y ejemplos de formato de etiquetas (Español)
Cord Blood Custom Labeling Solutions
We are your source for generating labels to identify cord blood unit bags and testing sections. Learn more about our custom formats and segement labels
v8.1 FACT-JACIE
International Standards for Hematopoietic Cellular Therapy
December 14, 2021
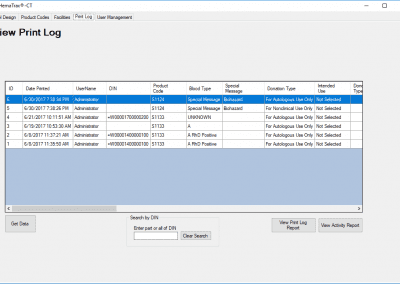
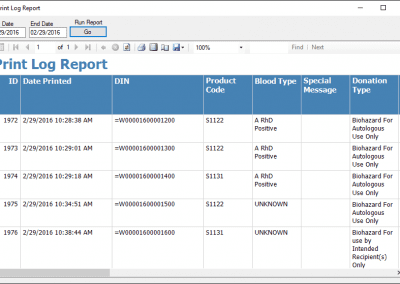
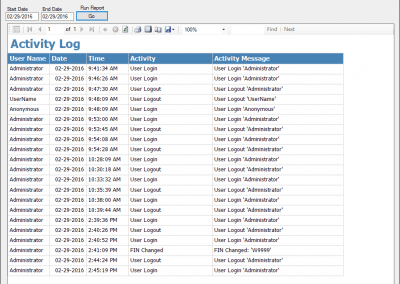
HemaTrax-CT Release Notes
Version 3.7.3
HemaTrax-CT User Guide
Version 3.7.0
HemaTrax-CT System Requirements
Version 3.7.0
HemaTrax-CT label gallery
View our blank ISBT 128 labels for use with the HemaTrax-CT system
Additional brochures
View all our Cellular Therapy documents



Now is the time to implement a simple, on demand print solution for ISBT 128 labeling. Use our HemaTrax®-CT cellular therapy product labeling system to ensure your facility meets these standards.
The new edition (version 8.1) (see pdf at right) of the FACT-JACIE International Standards for Hematopoietic Cellular Therapy was published December 14, 2021. Please note implementation of ISBT 128 labeling is required for FACT-JACIE accredidation effective May 20, 2018. Don’t be ISBT 128 non-compliant! Implementation of our HemaTrax®-CT system includes a printer, proprietary software, ribbons and labels— all required for ISBT 128 compliance.
HemaTrax-CT Brochure
View info and label format samples
HemaTrax-CT Folleto (Spanish)
Ver información y ejemplos de formato de etiquetas (Español)
v8.1 FACT-JACIE
International Standards for Hematopoietic Cellular Therapy
December 14, 2021
HemaTrax-CT label gallery
View our blank ISBT 128 labels for use with the HemaTrax-CT system
Phone
800-356-6126
Location
650 Heathrow Drive
Lincolnshire, IL 60069
Business Hours
M - F: 8:30 am - 5:00 pm CST
S - S: Closed
Will be used in accordance with our Privacy Policy.
Terms and Conditions